LumiThera Valeda ®
Harnessing the Power of Light to treat Dry Macular Degeneration (AMD)
LumiThera is a medical device company at the forefront in the development of photobiomodulation (PBM) treatment protocols for patients with dry Age-related macular degeneration (AMD), a leading cause of blindness in adults over 65.
What is AMD?
Age-related macular degeneration, or AMD, is an ocular disease characterised by cellular dysfunction in the macula resulting in significant visual impairment. Initially, the sharp, central vision is impacted but the progression of the disease can lead to complete blindness. While there are two forms of the disease, AMD starts with the dry form characterised by protein deposits called drusen that form on the retina (beneath the macula) causing degeneration of the cells over time.
What causes AMD?
The main cause of AMD is linked with aging which in influenced by genetic inheritance, race and even the pigmentation of your iris, lighter eyes being more at risk. There also environmental and lifestyle influences such and UV rays, high levels of pollution, smoking, abnormally high cholesterol and arterial hypertension.
Until now the management/treatment of dry AMD was limited to lifestyle advice which included taking supplements and UV protective eyewear. We can now add the LumiThera Valeda, the only approved treatment for Dry AMD.
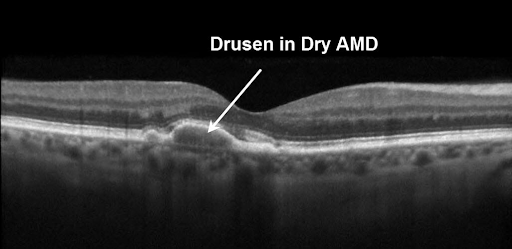
The Science
What Lumithera have developed is a light delivery system (LDS) that uses three wavelengths of light; these wavelengths have been proven to improve mitochondrial output and bioenergetics within the retinal tissues. This mechanism of photobiomodulation (PBM) works at a cellular level and has been ascribed to the activation of mitochondrial respiratory chain components resulting in stabilisation of metabolic function and initiation of a signalling cascade, which promotes cellular proliferation and cytoprotection. This improved bioenergetics/mitochondrial output has been shown to slow down the progression of Dry AMD in 80% of treated patients (Lightsite I - III studies) measured at thirteen months.
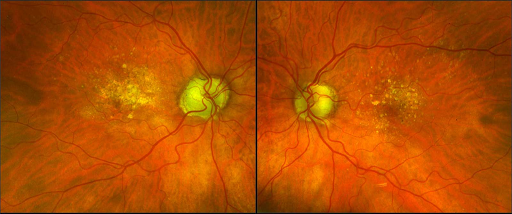
PMB is not only used by LumiThera for treatment of AMD, but was originally developed and used in physiotherapy, arthritis, wound repair and sports medicine and recently being recognised as a therapy for the treatment of serious, life-threatening disease states.
Treatment
The Valeda device is the only approved treatment to target dry AMD within the retina to slow down the progression of AMD. Each session lasts roughly 15 minutes to treat both eyes with the three different wavelengths. It is recommended to have 4 monthly cycles which consist of 9 sessions spaced evenly over a 3 week period.
The Valeda device is currently available in our Dore and Fleet Street practices. For more information on LumiThera Valeda and what it can do for you, please visit your local Eye Place, and speak to a member of our team today.
Pricing
| One Eye | Both Eyes | |
|---|---|---|
| Per treatment cycle | £800 | £1,500 |
| Direct Debit | £230 | £380 |
Terms
Per treatment includes Scans at the time of the treatment but not the recommended 6 monthly Eye Examination
Direct Debit includes 3 treatments cycles per year, Any Necessary Supplements, all scans and 6 monthly Eye Examination Scans at treatment cycle - Radial or 5 line Macula scan & Optomaps
Teatment Cycle - 9 light treatment sessions preferably over a 3 week period and recommended every 4 months
MacuShield/MacuShield Gold/Neutrof is part of the Direct Debit plan - price doesn’t change should it not be required
